

Researchers at the Henan Institute of Advanced Technology of Zhengzhou University (ZZU) have developed an innovative light-controlled cell encapsulation system for cancer immunotherapy. The study introduces a single engineered cell system regulated by red and far-red light, enabling precise spatiotemporal control of immune factor release within the body through a programmable, reversible, and low-toxicity system.
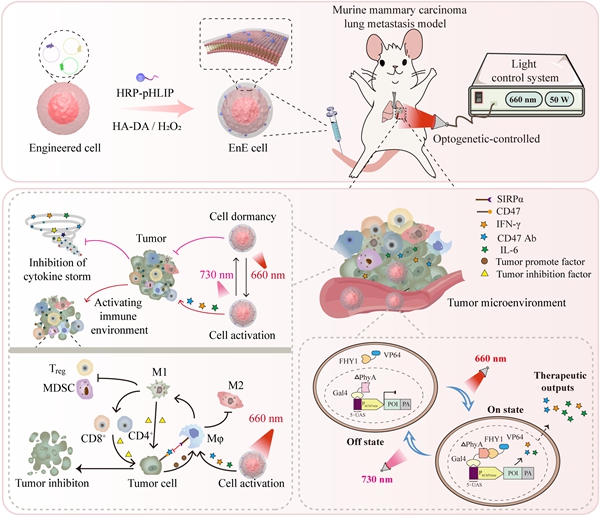
The schematic diagram illustrates the optogenetic cytokine immunotherapy system mediated by engineered EnE cells. [Photo/zzu.edu.cn]
The system addresses key challenges in engineered cell therapies, such as cytokine storms and off-target effects. It incorporates a plant-derived light-sensitive protein, ΔPhyA, which acts as a molecular switch. Under red light, ΔPhyA dimerizes with the FHY1 protein to trigger the expression of immune factors. Under far-red light, the dimer dissociates, halting protein production. This reversible mechanism allows remote, on-demand control of therapeutic activity.
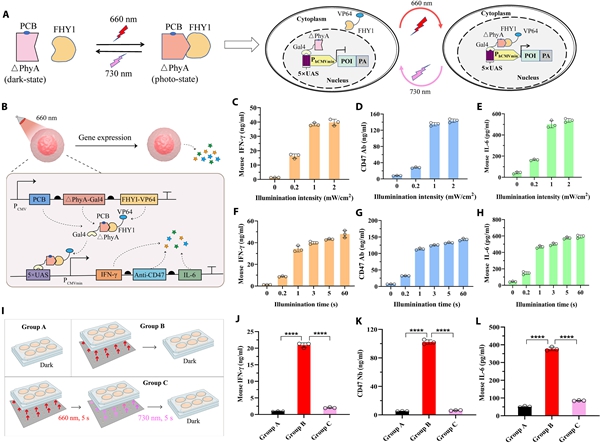
Construction and performance characterization of the optogenetic control system. [Photo/zzu.edu.cn]
In a mouse model of lung metastasis, the light-regulated system demonstrated strong anti-tumor efficacy without significant systemic toxicity. Unlike traditional cell therapies, it maintained high activity while improving safety, effectively decoupling treatment effectiveness from adverse effects. The approach transforms engineered cells into remotely controllable "living drug factories" within the body.
Published in journal Science Advances under the title "On-demand cancer immunotherapy via single-cell encapsulation of synthetic circuit-engineered cells", the study was led by Zhao Yue and Li Rui, co-first authors of the paper, with Chen Yazhou and Nie Guangjun as corresponding authors.
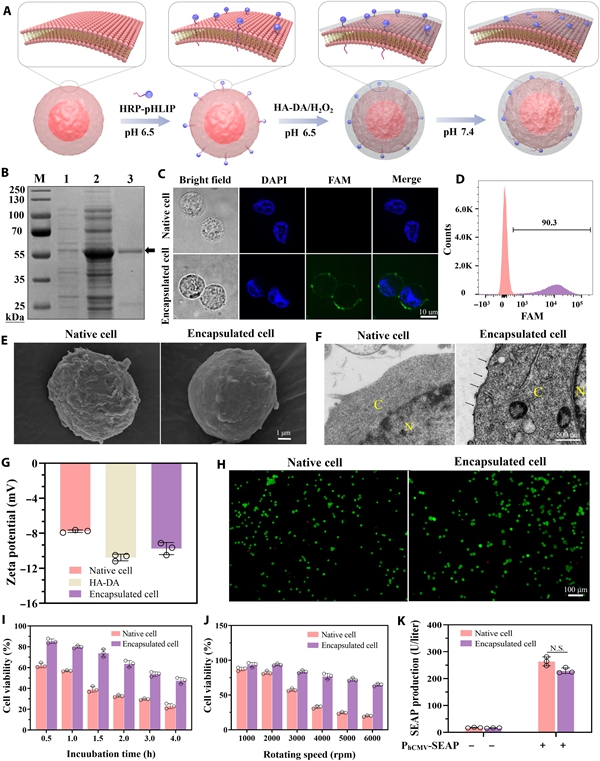
Engineering and characterization of encapsulated cell. [Photo/zzu.edu.cn]